Microglial research has emerged as a pivotal frontier in neuroscience, particularly in understanding neurodegenerative diseases such as Alzheimer’s. These remarkable microglial cells are integral to the brain’s immune system, constantly monitoring for damage and facilitating vital cellular processes like synaptic pruning. However, as recent studies led by experts like Beth Stevens reveal, this may also lead to detrimental outcomes when microglia misfunction, contributing to conditions like Alzheimer’s disease and Huntington’s disease. By investigating the complex role of microglia, researchers aim to develop new biomarkers and treatment strategies that could revolutionize how we approach these debilitating disorders. This ongoing research not only enhances our knowledge of brain health but also offers hope to the millions affected by neurodegenerative diseases.
Exploring the dynamics of glial cells, particularly their microglial counterparts, opens a new avenue for understanding brain health and its vulnerabilities to diseases. Known as the brain’s immune defenders, these cells play a crucial role in managing the brain’s responses to injury and disease, shaping neuronal connections vital for brain function. Insights from studies in this field, such as those by Beth Stevens, highlight the delicate balance microglial cells maintain in both protecting and potentially damaging neural networks. As researchers delve deeper into the behaviors of these immune cells, their findings have significant implications for tackling serious conditions like Alzheimer’s and other neurodegenerative illnesses. This exploration of glial biology is critical not only for advancing scientific knowledge but also for developing effective therapeutic interventions.
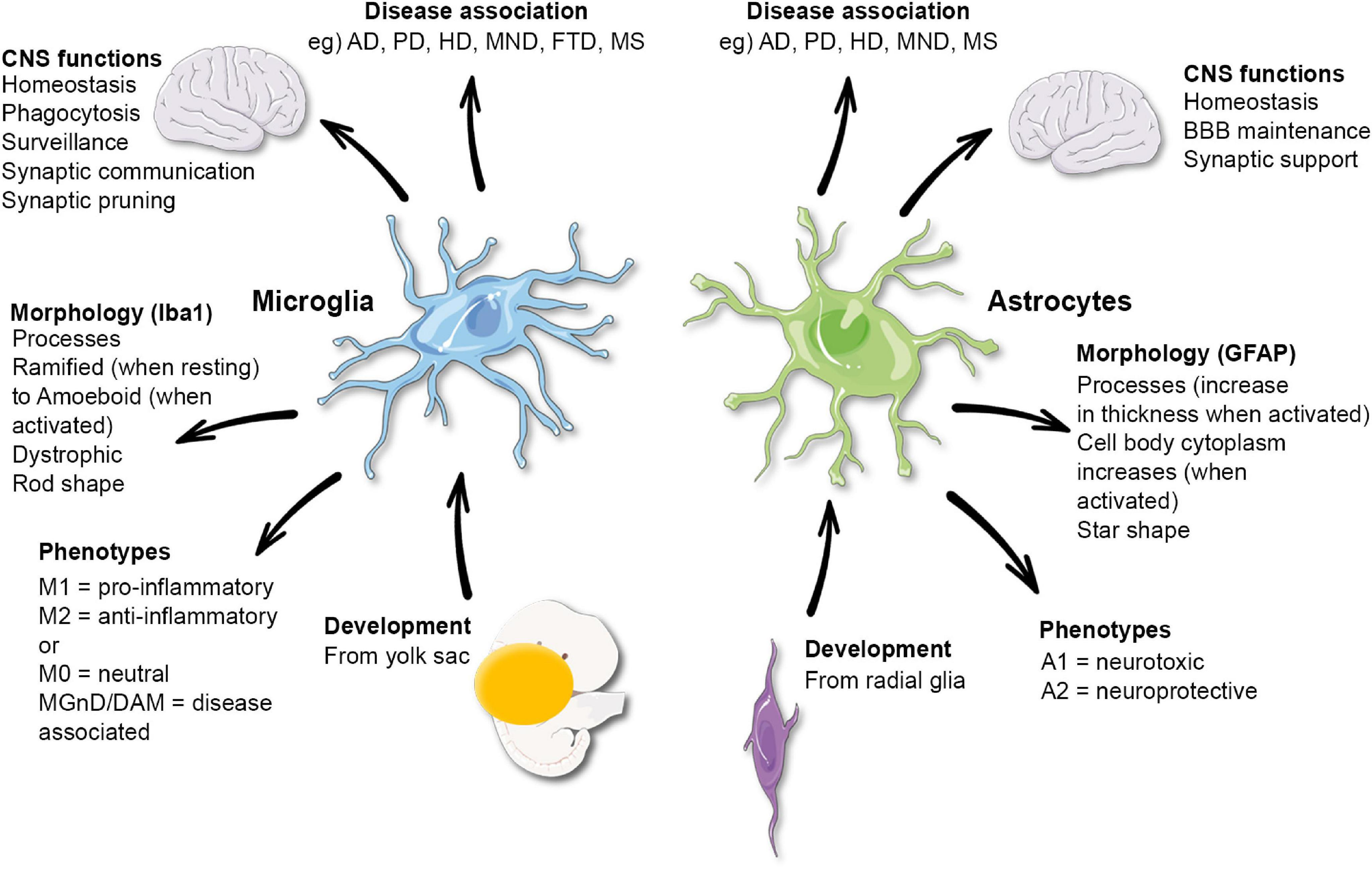
Understanding Microglial Cells: The Brain’s Immune Warriors
Microglial cells, often referred to as the brain’s immune cells, are essential for maintaining brain health. They play a vital role in monitoring the central nervous system and responding to injuries by eliminating debris and damaged neurons. This function is crucial in the prevention of neurodegenerative diseases, such as Alzheimer’s disease, where faulty microglial activity can lead to increased synaptic pruning. Understanding how microglial cells function provides valuable insight into potential therapeutic targets for a variety of neurological disorders.
Research led by Beth Stevens has illuminated the complexities of microglial behavior, especially during developmental stages. These cells not only respond to pathological changes but also actively participate in shaping synaptic connections in the brain. Their dual role highlights the importance of microglia, not just as responders to injury but as vital regulators in brain homeostasis. Furthermore, Stevens’ work underscores how imbalances in microglial activity can result in harmful outcomes, paving the way for developing strategies to mitigate these risks in conditions like Alzheimer’s.
The Link Between Microglial Dysfunction and Neurodegenerative Diseases
The connection between microglial dysfunction and the progression of neurodegenerative diseases is a growing area of interest in neuroscience. As affected cells in the brain, microglia play a crucial role in regulating inflammation and immune responses. In diseases such as Alzheimer’s, dysfunctional microglia can lead to excessive cleaning of synapses, which can contribute to neuronal loss and cognitive decline. Investigative efforts, including those from Stevens Lab, have focused on understanding the pathways involved in microglial activation and how these processes can become misregulated.
Understanding the mechanisms of microglial dysfunction will enable researchers to identify biomarkers for early detection of neurodegenerative diseases. This could have significant implications for treatment strategies aimed at modulating microglial activity or enhancing their protective responses. The pioneering work of Beth Stevens in this domain has laid the groundwork for innovative research that seeks to transform our approach to combating these debilitating conditions.
Beth Stevens: A Pioneer in Microglial Research
Beth Stevens has emerged as a leading figure in microglial research, significantly advancing our understanding of these unique immune cells. Her extensive studies on microglial function have not only contributed to the foundational knowledge of their role in brain development but also revealed their involvement in neurodegenerative diseases. As she puts it, ‘Our microglial research is a prime example of an immune-related pathway that we would never have been able to advance without foundational science.’ This statement underscores the critical importance of curiosity-driven exploration in generating tangible knowledge.
Through meticulous experimentation, her lab at Boston Children’s Hospital and the Broad Institute has opened new avenues for therapeutic intervention. Stevens emphasizes that the implications of microglial research extend far beyond basic biology, potentially leading to breakthroughs in treatment strategies for Alzheimer’s disease and beyond. With the ongoing support from the NIH and federal agencies, her team continues to innovate, finding answers to complex questions surrounding the brain’s immune system.
Potential Biomarkers for Alzheimer’s Disease from Microglial Studies
The exploration of microglial cells has led to significant advancements in identifying potential biomarkers for Alzheimer’s disease. By understanding how microglia react to pathological stimuli, researchers can elucidate specific changes in cell behavior that signify the onset of neurodegeneration. Stevens’ research into the interactions between microglial function and synaptic integrity is crucial in this respect, as early detection of these pathological changes could lead to timely interventions.
Furthermore, Stevens and her team are investigating how the alterations in microglial activity may correlate with cognitive decline in individuals at risk for Alzheimer’s. This intersection of basic science and clinical application is pivotal, suggesting that profiling microglial health could offer predictive insights into the progression of Alzheimer’s. In doing so, the findings could revolutionize how clinicians monitor at-risk populations and implement preventative strategies.
Innovative Approaches to Modulating Microglial Activity
Emerging research has shifted focus towards modulating microglial activity as a therapeutic avenue to combat neurodegenerative conditions. Since these cells are crucial for maintaining brain health, developing strategies that enhance their beneficial functions or dampen their harmful effects could be groundbreaking. Beth Stevens’ innovative research seeks to uncover methodologies to recalibrate microglial responses, ensuring they support neuronal health instead of contributing to degeneration.
Modulating microglial activity can also involve pharmacological interventions that target specific signaling pathways. By refining our understanding of these pathways through foundational research, scientists can develop tailored therapies that hold promise for conditions like Alzheimer’s and Huntington’s disease. Ultimately, the goal is to harness the protective capabilities of microglia while preventing their involvement in pathological processes, marking a significant shift in treating neurodegenerative diseases.
The Role of Synaptic Pruning in Brain Health
Synaptic pruning is a natural process wherein microglial cells remove excess synapses, supporting the overall plasticity of the brain. This process is critical during brain development and is believed to be essential for learning and memory. However, abnormal synaptic pruning by microglia has been implicated in the development of neurodegenerative diseases, including Alzheimer’s. Beth Stevens’ research highlights that when microglia become overly aggressive in pruning, they can inadvertently accelerate synaptic loss, leading to cognitive impairments.
Understanding the delicate balance of synaptic pruning provides insight into potential therapeutic targets. If researchers can identify the signals that drive microglial cells to prune synapses too aggressively, they may be able to develop treatments that prevent this harmful activity. This could have profound implications not only for Alzheimer’s disease but for enhancing our understanding of how microglial activity influences brain health throughout one’s life.
Functional Implications of Microglial Research
Microglial research extends well beyond academic curiosity; it has practical implications for improving human health. Understanding how these immune cells operate enables scientists to draw connections between immune responses and neurodegenerative diseases. By studying microglia, researchers have the potential to design interventions that manipulate these cells to promote healing in the brain. Beth Stevens’ work illustrates how foundational research can lead to real-world applications, impacting lives and treatment protocols for those suffering from Alzheimer’s.
As microglial research continues to evolve, it holds the promise of unveiling novel therapeutic avenues. Insights gained from Stevens’ research may soon translate into strategies that not only halt disease progression but also enhance the quality of life for millions impacted by neurodegenerative disorders. The ability to target specific molecular pathways within microglia could redefine treatment paradigms in neurology, emphasizing preventative care as a cornerstone of managing Alzheimer’s disease and related conditions.
Federal Funding and Its Impact on Microglial Research
Federal funding plays a pivotal role in advancing scientific inquiry, particularly in fields like microglial research. As Beth Stevens articulated, the robust support from agencies such as the NIH has been crucial for her lab’s progress in understanding how microglial dysfunction contributes to neurodegenerative diseases. Without such backing, researchers might struggle to pursue comprehensive studies that require extensive resources and long-term commitment.
The impact of federal funding extends beyond the laboratory. It fosters a collaborative environment where scientists can share findings, cultivate new ideas, and accelerate the pace of discovery. This supportive infrastructure is vital for navigating complex problems like Alzheimer’s disease, where understanding the immune mechanisms in the brain can lead to innovative pharmaceutical developments and ultimately improve patient outcomes.
Future Directions in Microglial Cell Research
The future of microglial research is poised for significant advancements, driven by ongoing discoveries and technological innovations. As researchers like Beth Stevens continue to explore the intricacies of microglial function, we can expect to see a paradigm shift in how neurodegenerative diseases are understood and treated. Future studies will likely focus on the molecular mechanisms that govern microglial behavior, looking for novel ways to manipulate their functions to maximize neuroprotection while minimizing inflammation.
Additionally, as the field of neuroimmunology expands, integrating knowledge from various research fronts will be crucial. Collaborations across disciplines can enhance our understanding of how microglial cells interact with other immune cells and peripheral signals influencing brain health. This multifaceted approach towards studying microglia may unlock new therapeutic strategies, ultimately leading to breakthroughs that could benefit millions affected by Alzheimer’s disease and other neurodegenerative disorders.
Frequently Asked Questions
What role do microglial cells play in neurodegenerative diseases like Alzheimer’s disease?
Microglial cells are crucial components of the brain’s immune system, monitoring brain health and responding to injury or disease. In conditions like Alzheimer’s disease, these cells can become overactive or dysfunctional, leading to abnormal pruning of synapses. This abnormal activity is associated with the progression of neurodegenerative diseases, making microglial research vital for understanding and potentially treating these conditions.
How does Beth Stevens’ research impact our understanding of Alzheimer’s disease?
Beth Stevens’ research sheds light on the functions of microglial cells in the brain, particularly their role in synaptic pruning. Her findings indicate that dysregulation in this process may contribute to Alzheimer’s disease and other neurodegenerative disorders. Her work is pioneering in identifying new biomarkers and therapies aimed at correcting the harmful activities of microglia, which could improve treatment outcomes for patients.
Why are microglial cells considered part of the brain’s immune system?
Microglial cells act as the first line of defense in the brain’s immune system. They continuously survey brain tissue for signs of injury, inflammation, or disease. By removing debris and modulating synaptic connections, microglia play a critical role in maintaining brain health and function, particularly in neurodegenerative diseases such as Alzheimer’s.
What are the implications of abnormal microglial function in neurodegenerative diseases?
Abnormal microglial function can lead to excessive synaptic pruning and increased inflammation, which are linked to the progression of neurodegenerative diseases like Alzheimer’s and Huntington’s disease. Understanding these processes through microglial research is essential in developing targeted therapies that restore normal function and potentially slow disease progression.
How does foundational microglial research contribute to advancements in treatment for Alzheimer’s disease?
Foundational microglial research, such as that conducted by Beth Stevens, provides critical insights into the mechanisms of disease. By exploring how microglial cells interact with neurons and contribute to synaptic health, researchers can identify new therapeutic targets. This research lays the groundwork for developing innovative treatments that aim to modify the disease process in Alzheimer’s patients.
What are the potential therapeutic targets identified in microglial research for treating Alzheimer’s disease?
Recent microglial research has identified various potential therapeutic targets, including pathways involved in synaptic pruning and inflammation regulation. Modulating these pathways could restore normal microglial function and improve brain health in Alzheimer’s disease, offering hope for more effective treatments.
Can microglial research lead to biomarkers for Alzheimer’s disease?
Yes, microglial research is crucial in identifying biomarkers for Alzheimer’s disease. By understanding the specific roles and changes in microglial activity during disease progression, researchers can develop biomarkers that may offer early detection and improve monitoring of Alzheimer’s, enhancing patient care.
What is the significance of the National Institutes of Health (NIH) funding in microglial research?
NIH funding is pivotal for advancing microglial research, providing the financial resources necessary for scientists to explore complex questions related to brain immunity and neurodegeneration. This support allows researchers like Beth Stevens to conduct innovative studies that could lead to breakthroughs in understanding and treating diseases like Alzheimer’s.
| Key Research Focus | Findings | Impact | Quote |
|---|---|---|---|
| Microglial research by Beth Stevens | Microglia act as the brain’s immune system, pruned synapses vital for neuron communication | Research aids in understanding Alzheimer’s and other neurodegenerative diseases | “Our microglial research…would never have been able to advance without foundational science and curiosity-driven research.” |
| Stevens Lab’s objectives | Abnormal pruning linked to Alzheimer’s, Huntington’s, and other disorders | Potential new biomarkers and treatments for millions of affected Americans | “The foundation of our research…was almost entirely driven by the National Institutes of Health and other federal funding.” |
| Evolution of microglial research | Discoveries stem from curiosity and foundational science leading to unexpected insights | Research contributes to broader understanding and white insights into disease mechanisms | “I was just following the science…It was this fascinating new area.” |
Summary
Microglial research has become a crucial area of study in understanding diseases such as Alzheimer’s. Beth Stevens’ work significantly advances our comprehension of how microglia function within the brain, acting as guardians against disease while also revealing the intricate balance required for healthy brain function. The findings hold promise for groundbreaking treatments and biomarkers, underscoring the importance of curiosity-driven science in uncovering the complexities of neurological disorders.