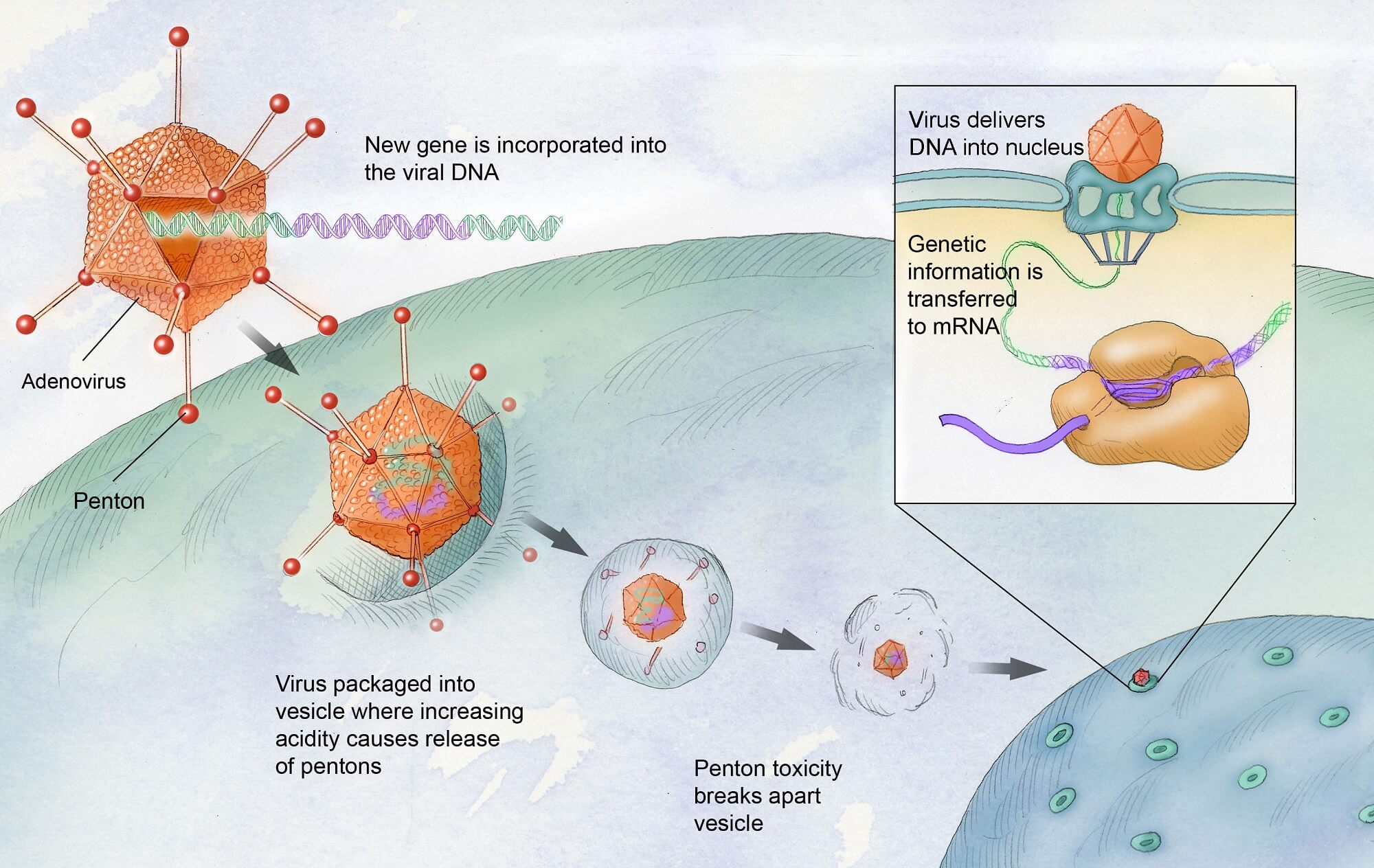
Gene therapy for hemophilia represents a groundbreaking advancement in treating this inherited bleeding disorder. Historically, individuals with hemophilia, particularly hemophilia B, relied heavily on regular clotting factor therapy to manage their condition and prevent spontaneous bleeding. With the recent FDA approval of Hemgenix, patients now have a transformative option that may significantly reduce or eliminate the need for ongoing treatments. This innovative gene therapy offers the potential for lasting effects, providing patients with a newfound sense of hope and possibility. The benefits of gene therapy for hemophilia extend beyond mere physical health, promising a better quality of life free from the constant worry of bleeding episodes and injection schedules.
The evolution of treatment for hemophilia, especially through advanced gene modifications, is leading to a new era of care for individuals diagnosed with this condition. This innovative approach, often referred to as genetic intervention, aims to correct the underlying deficiencies in blood components, specifically by enhancing the production of clotting factors. The recent licensing of treatments like Hemgenix marks a significant milestone, highlighting a shift in how hemophilia can be effectively managed. Years of research have culminated in this promising therapy, which not only presents potential cures for hemophilia B but also embodies a broader spectrum of gene therapy possibilities. As we explore these advanced treatment options, the future looks brighter for those who have long struggled with the challenges of managing their hemophilic conditions.
Understanding Gene Therapy for Hemophilia
Gene therapy for hemophilia represents a groundbreaking advance in treatment options for patients suffering from this genetic blood disorder. By targeting the specific genetic mutations that cause hemophilia, new therapies like Hemgenix work by introducing a correct copy of the defective gene into the patient’s liver cells. This innovative approach not only aims to provide a long-lasting solution to the deficiency of clotting factor IX but also reduces the dependence on traditional clotting factor therapy, which often requires frequent injections and can be cumbersome for patients. As research continues to evolve, gene therapy stands as a beacon of hope for those like Terence Blue, who have spent their lives navigating the challenges posed by hemophilia.
The implications of gene therapy extend beyond mere treatment; they highlight the potential for a cure that could drastically improve the quality of life for hemophilia patients. With Hemgenix approved by the FDA and its encouraging clinical trial results, it signifies a promising shift in how hemophilia is managed. Patients may experience fewer spontaneous bleeds and potentially avoid lifelong dependency on clotting factor injections. The expectation is that these therapies will reframe the narrative around hemophilia, shifting from management and mitigation towards actual healing and restoration of normal functioning.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia is a revolutionary treatment that aims to correct the genetic mutation responsible for the disease. In hemophilia B, for instance, the therapy involves introducing a corrected copy of the clotting factor IX gene into the liver using a modified virus. This process enables the liver to produce sufficient amounts of the necessary clotting factor, potentially reducing or eliminating the need for regular clotting factor therapy.
What are the benefits of gene therapy for hemophilia compared to traditional treatments?
Gene therapy for hemophilia offers several significant benefits over traditional treatments, such as clotting factor therapy. With gene therapy, patients may experience long-lasting effects, reducing the frequency of infusions. The approved therapy, Hemgenix, has shown promising results, where many patients can maintain adequate levels of factor IX without ongoing treatments, leading to better quality of life.
What is Hemgenix and what has it achieved for hemophilia B patients?
Hemgenix is a groundbreaking gene therapy approved by the FDA for treating hemophilia B. This therapy aims to provide a long-term solution by enabling the body to produce factor IX, the protein missing in individuals with hemophilia B. Early results indicate that around 94% of patients treated with Hemgenix did not require additional prophylaxis three years after treatment.
Is gene therapy for hemophilia B considered a cure?
While gene therapy, such as Hemgenix, has the potential to significantly reduce or eliminate the need for regular clotting factor treatments, it is cautious to describe it as a complete ‘cure.’ It has demonstrated the ability to provide lasting effects on hemophilia B symptoms, leading to improved clotting factor production and decreased reliance on traditional therapies.
How will gene therapy impact the future treatment landscape for hemophilia?
The emergence of gene therapy for hemophilia represents a significant shift in treatment paradigms. As therapies like Hemgenix gain traction, they could reshape care by offering long-term solutions that may reduce healthcare costs associated with chronic medication use. This innovation encourages further research and development, promising new therapies that address other forms of hemophilia and related disorders.
What are the primary challenges facing gene therapy for hemophilia?
Despite its potential, gene therapy for hemophilia faces several challenges, including high costs of treatment, limited patient uptake, and market pressures. For example, Hemgenix is priced at approximately $3.5 million, which can be a barrier for many patients. Additionally, ensuring healthcare providers and patients fully understand and accept this new approach is crucial for its success.
What kind of outcomes can patients expect after receiving gene therapy for hemophilia?
Patients receiving gene therapy for hemophilia, particularly hemophilia B, can expect varying outcomes. Many may achieve significant improvements in their ability to produce factor IX, leading to fewer spontaneous bleeds and a reduced need for prophylactic treatments. Clinical trials have shown that a majority of treated patients no longer require frequent clotting factor infusions after therapy.
How does gene therapy for hemophilia compare to other advanced treatment options?
Gene therapy for hemophilia stands out among advanced treatment options due to its potential for long-term effects with a single administration. Unlike regular infusions associated with clotting factor therapy, gene therapy targets the root cause of hemophilia by correcting genetic deficiencies. This positions it as a landmark development within the broader field of genetic and cell therapies.
What is the process of receiving gene therapy for hemophilia like?
Receiving gene therapy for hemophilia typically involves an outpatient procedure. For example, during Hemgenix administration, a patient’s liver is targeted with a virus carrying the corrected gene. The entire process may take a few hours, and patients are monitored closely for any side effects. Many patients report minimal discomfort and experience significant improvements post-treatment.
Are there any side effects associated with gene therapy for hemophilia?
While gene therapy for hemophilia has shown promise, some patients may experience side effects such as elevated liver enzymes. These effects are usually manageable and temporary. Medical teams monitor patients closely during and after treatment to mitigate risks and ensure the best possible outcomes.
| Key Points |
|---|
| Terence Blue became the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B, in February 2025. |
| Hemophilia is a genetic disorder where blood does not clot properly, commonly affecting males. |
| Gene therapy aims to introduce a corrected copy of the gene to produce the missing clotting factor. |
| The treatment was developed by CSL Behring and FDA approved in November 2022. |
| The cost of the therapy is approximately $3.5 million per patient. |
| Early results show promise, with the majority of participants in clinical trials not requiring ongoing treatments. |
| Gene therapies face market pressures and challenges regarding patient acceptance and insurance coverage. |
Summary
Gene therapy for hemophilia offers groundbreaking potential for patients like Terence Blue who seek relief from daily management burdens. As the first patient in New England to receive the Hemgenix treatment, Blue’s experience is a beacon of hope in the evolving field of gene therapy. This innovative approach not only seeks to change the lives of individuals suffering from hemophilia but also represents a significant milestone in medical science, offering new avenues for healing where traditional treatments fell short. With continued advancements and potential for market expansion, gene therapy could redefine the future of hemophilia care.